Discovery May Help Explain Immunotherapy Resistance, Hints at New Therapies
Immunotherapy drugs known as checkpoint inhibitors have revolutionized cancer treatment: many patients with malignancies that until recently would have been considered untreatable are experiencing long-term remissions. But the majority of patients don’t respond to these drugs, and they work far better in some cancers than others, for reasons that have befuddled scientists. Now, UC San Francisco researchers have identified a surprising phenomenon that may explain why many cancers don’t respond to these drugs, and hints at new strategies to unleash the immune system against disease.
“In the best-case scenarios, like melanoma, only 20 to 30 percent of patients respond to immune checkpoint inhibitors, while in other cases, like prostate cancer, there is only a single-digit response rate,” said Robert Blelloch, MD, PhD, professor of urology at UCSF and senior author of the new study, published April 4 in Cell. “That means a majority of patients are not responding. We wanted to know why.”
In malignant tissue, a protein called PD-L1 functions as an “invisibility cloak”: by displaying PD-L1 on their surfaces, cancer cells protect themselves from attacks by the immune system. Some of the most successful immunotherapies work by interfering with PD-L1 or with its receptor, PD-1, which resides on immune cells. When the interaction between PD-L1 and PD-1 is blocked, tumors lose their ability to hide from the immune system and become vulnerable to anti-cancer immune attacks.
One reason that some tumors may be resistant to these treatments is that they do not produce PD-L1, meaning that there is nowhere for existing checkpoint inhibitors to act — that is, they may avoid the immune system using other checkpoint proteins yet to be discovered. Scientists have previously shown the PD-L1 protein to be present at low levels, or completely absent, in tumor cells of prostate cancer patients, potentially explaining their resistance to the therapy.
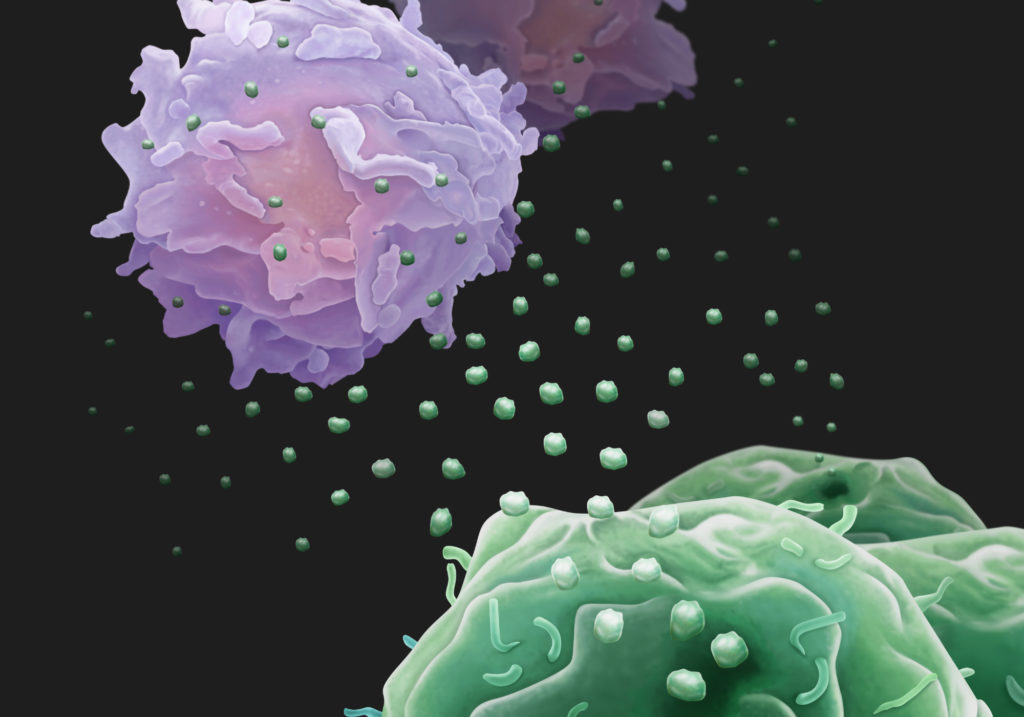
But in their new paper Blelloch’s group is suggesting a very different answer to this puzzle: PD-L1 is being mass-produced by these tumors, they found, but instead of displaying the protein on their surface, cancer cells export PD-L1 in molecular freighters known as exosomes. These PD-L1–packed exosomes sprout from cancer cells and travel through the lymphatic system or bloodstream to lymph nodes, the sites where immune cells are activated to protect the body. There, the PD-L1 proteins act as itinerant molecular saboteurs, remotely disarming immune cells and preventing them from locating tumors to mount an anti-cancer offensive.
So rather than shutting down the immune response at the tumor surface, exosomal PD-L1 can inhibit immune cells before they even arrive there. And unlike PD-L1 found on the tumor’s surface, exosomal PD-L1, for unclear reasons, is resistant to existing checkpoint inhibitors.
“The standard model says that PD-L1 acts on immune cells that travel to the tumor niche, where they encounter this immune-suppressing protein,” Blelloch said. “Our data suggests that this isn’t true for many immunotherapy-resistant tumors. These tumors evade the immune system by delivering exosomal PD-L1 to lymph nodes, where they inhibit the activation of immune cells remotely. These findings represent a break from dogma.”
Blelloch’s group decided to explore exosomes when they noticed something strange that suggested the standard model of PD-L1 presentation was flawed. Like scientists that came before, they found low levels of PD-L1 protein in resistant cancers. But when they looked at messenger RNA (mRNA), the molecular precursor of all proteins, they observed an odd discrepancy: there was far too much PD-L1 mRNA for the scant amount of PD-L1 protein that they measured in the cells.
“We saw the difference between mRNA and protein levels and wanted to figure out what was happening,” Blelloch said. “Our experiments also showed that the protein was in fact being made at some point, and that it wasn’t being degraded. That’s when we looked at exosomes and found the missing PD-L1.”
Exosomal PD-L1 Hampers Immune Response, Promotes Cancer Growth
To show that exosomal PD-L1 was responsible for imparting immune invisibility, the researchers turned to a mouse prostate cancer model that’s resistant to checkpoint inhibitors. When they transplanted these cancer cells into healthy mice, tumors rapidly sprouted. But when the scientists used the gene-editing tool CRISPR to delete two genes required for exosome production, the edited cancer cells were unable to form tumors in genetically identical mice. Though both edited and unedited cells were producing PD-L1, only those unable to create exosomes were visible and vulnerable to the immune system when PD-L1 was blocked.
“The importance of this discovery was immediately evident,” said postdoctoral fellow Mauro Poggio, PhD, lead author of the new study. “Currently in the clinic, there are no drugs available that are capable of counteracting the destructive power of exosomal PD-L1, so understanding the biology of exosomal PD-L1 is the first fundamental step that might lead to novel therapeutic approaches for patients.”
In a complementary experiment, the same CRISPR-edited cancer cells were transplanted into healthy mice, immediately followed by a series of injections of exosomes carrying PD-L1. Unable to produce exosomes, the CRISPR-edited cancer cells should have fallen victim to the immune system. Instead, the injected exosomes were able to neutralize the immune response on behalf of the cancer, which allowed the exosome-deficient cancer cells to form tumors.
To figure out how exosomal PD-L1 was interfering with the immune system, the researchers inspected the lymph nodes of mice that received either CRISPR-edited or unadulterated cancer cells. Mice that received the edited cells showed increased immune cell proliferation and had higher numbers of activated immune cells in their lymph nodes, the central command hubs of the immune system.
In a separate mouse model — a colorectal cancer that’s only partially responsive to immunotherapy — the researchers identified two distinct pools of PD-L1: one on the surface of tumor cells that’s sensitive to PD-L1 inhibitors, and another in exosomes that’s resistant. When they treated the cancer with a combination therapy that involved both preventing exosome formation and administering PD-L1 inhibitors, the mice survived longer than those treated with either approach alone.
“These data from two very different cancer models suggest a novel therapeutic approach, where suppressing the release of PD-L1 in exosomes, either alone or in combination with current checkpoint inhibitors, could overcome resistance in a large fraction of patients currently resistant to treatment with checkpoint inhibitors alone,” Blelloch said.
Exosome-Deficient Tumor Cells Can Act as ‘Vaccine’ Against Immune Resistance
In a surprising result from the new paper, the researchers found that they could use CRISPR-edited, exosome-deficient cancer cells to induce an anti-cancer immune response that targeted tumors that normally resist immune attack.
The researchers first transplanted CRISPR-edited cancer cells unable to produce exosomes into normal mice and waited 90 days. They then transplanted unedited — and presumably immune-evading — cancer cells into the same mice. After having exposed the immune system to the CRISPR-edited, exosome-deficient cancer cells, the unedited cells were no longer invisible. Instead of ignoring these cells, the immune system mounted a vigorous response that targeted these formerly immune-evading cancer cells and prevented them from proliferating.
“The immune system develops an anti-tumor memory after being exposed to cancer cells that can’t produce exosomal PD-L1. Once the immune system has developed memory, it is no longer sensitive to this form of PD-L1 and thus targets exosomal PD-L1–producing cancer cells as well,” Blelloch said.
Another surprising result was achieved when both unedited and CRISPR-edited, exosome-deficient cancer cells were simultaneously transplanted into opposite sides of the same mouse. Though they were introduced at the same time, the CRISPR-edited cells proved dominant — they were able to activate the immune system, which then launched an attack that destroyed the unedited, supposedly immune-resistant tumors growing on the other side.
These results suggest that even the temporary inhibition of the release of PD-L1 in exosomes could lead to long-term, body-wide suppression of tumor growth. Furthermore, they hint at the possibility of a new kind of immunotherapy, one in which a patient’s cancer cells can be edited and reintroduced in order to activate the immune system and goad it into attacking immune-resistant cancers. Suppressing the release of PD-L1 in exosomes or the introduction of the “tumor cell vaccine” devised by the Blelloch team may one day offer hope to patients whose tumors don’t respond to today’s treatment options.
“Much more needs to be uncovered about PD-L1’s function in cancer,” Poggio said. “We are just scratching the surface of what could be a new mechanism that, if blocked, has the potential to suppress many aggressive tumors that don’t currently respond to treatment.”
Authors: Additional authors on the paper include TJ Hu, Chien-Chun Pai, Brandon Chu, Cassandra D. Belair, Anthony Chang, Ursula E. Lang, Qi Fu, and Lawrence Fong of UCSF; Elizabeth Montabana of UC Berkeley.
Funding: Research was supported by the National Institutes of Health Common Fund Extracellular RNA Consortium, the George and Judy Marcus Innovation Fund, and an NIH training grant.
Conflicts: The authors declare no competing financial interests.
About UCSF: UC San Francisco (UCSF) is a leading university dedicated to promoting health worldwide through advanced biomedical research, graduate-level education in the life sciences and health professions, and excellence in patient care. It includes top-ranked graduate schools of dentistry, medicine, nursing and pharmacy; a graduate division with nationally renowned programs in basic, biomedical, translational and population sciences; and a preeminent biomedical research enterprise. It also includes UCSF Health, which comprises three top-ranked hospitals – UCSF Medical Center and UCSF Benioff Children’s Hospitals in San Francisco and Oakland – as well as Langley Porter Psychiatric Hospital and Clinics, UCSF Benioff Children’s Physicians and the UCSF Faculty Practice. UCSF Health has affiliations with hospitals and health organizations throughout the Bay Area. UCSF faculty also provide all physician care at the public Zuckerberg San Francisco General Hospital and Trauma Center, and the SF VA Medical Center. The UCSF Fresno Medical Education Program is a major branch of the University of California, San Francisco’s School of Medicine. Please visit ucsf.edu/news.