The American Association of Extracellular Vesicles Annual Meeting will be held in Houston, Texas from November 10th-13th, 2024.
Early bird registration ends September 1st!
For more information please visit 2024 Houston | AAEV


08.16.24
Common Fund Data Ecosystem (CFDE) Virtual Workshop:
“Biomarker discovery from extracellular (cell-free) RNA-seq and tissue of origin from GTEx RNA-seq profiles”
Thursday September 12th 2024
1-3 pm ET / 10am-Noon PT
Requires Free Registration by September 9th (below)
Join us for an engaging hands-on workshop where Srimeenakshi (Meenu) Srinivasan of the Louise Laurent Lab at the University of California-San Diego will demonstrate how to perform integrative data analysis utilizing data from two Common Fund Data Ecosystem (CFDE) Data Coordinating Center portals followed by an interactive Q&A session.
The featured use case will illustrate biomarker discovery from extracellular cell-free RNA-seq data with tissue of origin identification through integrative analysis of GTEx tissue-derived RNA-seq data and plasma-derived small RNA-seq data from the ERCC exRNA Atlas.
To conduct workshop exercises in real time, attendees wishing to take part in the hands-on analysis will need to have the following installed prior to dialing into the workshop as there will not be time to conduct installations during the workshop.
We also strongly encourage attendees to have Jupyter Notebook installed as well as this will be the platform used during the workshop. The Jupyter Notebook/files that will be used will be sent out via email.
Attendees who are not looking to conduct the analyses during the workshop are welcome to attend and observe.
A link to the virtual meeting will be provided in a confirmation email.
If you have any questions, please feel free to contact us at info@exrna.org
We look forward to seeing you there!
To learn more about the projects & resources that will be discussed please see:
Common Fund Data Ecosystem (CFDE): https://commonfund.nih.gov/dataecosystem
Extracellular RNA Communication Consortium (ERCC): https://exrna.org/
Genotype-Tissue Expression Project (GTEx): https://www.gtexportal.org/
Registration and more information:
When: October 17 – 19, 2024
Where: Bethesda North Marriott Hotel & Conference Center
Abstracts are due August 30, 2024
Registration deadline: September 25, 2024
The ASIC Annual Meeting is an intellectual “home” and support network for new and seasoned investigators, students, and postdocs. Over three days you’ll exchange ideas on emerging questions and cutting-edge developments in non-EV research including:
Extracellular Vesicles (EVs)
Extracellular Particles (EPs)
Particulate carriers of extracellular RNA (exRNA) as biological mediators, regulators, and diagnostic analytes.
The meeting also covers a broad range of intercellular communication processes including:
EVs
EPs
exRNA in Cancer
CNS Diseases
Infections (both bacterial and viral)
WHY ATTEND?
You’ll have a valuable opportunity to network with investigators from diverse scientific and clinical fields, discuss and advance the impact of EV/EP/ExRNA in diagnostics and treatments, and better understand the biogenesis of normal vs. disease states.
Are you new to the field? You can work with colleagues and mentors so you can improve your grants and develop their skills to become successful independent researchers.
Women and those from underrepresented groups are especially encouraged to attend and connect with seasoned researchers who have years of mentoring experience.
11.23.20
The ASEMV2020 organizing committee would like to congratulate the winners of this year’s Young Investigator Awards. There were three speaker awards, for talks by a Young Investigator, a postdoctoral scholar, and a Ph.D. candidate. There are also two poster winners.

Moran Amit
Assistant Professor
Department of Head and Neck Surgery – Research
Division of Surgery
University of Texas MD Anderson Cancer Center
for work on the role of p53 and axonogenesis in cancer

Frederik Verweij
Post-Doctoral Fellow
Team van Niel
Institute of Psychiatry and Neuroscience of Paris
for research on EV biology in a zebrafish model system
See Dr. Verweij’s recent #WebEVTalk outlining the zebrafish model system for tracking EVs.

Hannah McMillan
Ph.D. Candidate
Kuehn Lab
Department of Molecular Genetics and Microbiology
Duke University
for studies on the protective immune pathways in plants elicited by bacterial OMVs

Killian O’Brien
Post-Doctoral Fellow
Breakefield Lab
Harvard Medical School &
Massachusetts General Hospital
for research on understanding the intracellular fate of EV-delivered content
 Kathleen Lennon
Kathleen Lennon
Ph.D. Candidate
Talisman Lab
Irell and Manella Graduate School of Biological Sciences
City of Hope
for work on EV characterization using quantitative Single Molecule Localization Microscopy (qSMLM)
This post originally appeared in Bioquick News.
The 2018 annual meeting of the American Society for Exosomes and Microvesicles (ASEMV) was held October 20-24 in Baltimore, hard by the water’s edge in the Baltimore Marriott Waterfront Conference Center. ASEMV president, Stephen Gould, PhD, Professor of Biological Chemistry & Co-Director, Graduate Program in Biological Chemistry, Johns Hopkins, reported a record attendance of 250 scientists from the United States and around the world (Korea, Norway, Sweden, Canada, Australia, Japan, UK, Italy, Portugal, The Netherlands) at this historically intimate and highly interactive meeting that benefits greatly from having communal meals and no overlapping sessions. The five-day meeting featured over 100 podium presentations and myriad posters. The daily consecutive sessions typically ran from 8.30 in the morning to 9.30 in the evening, and were followed by two hours of poster viewing and interaction among researchers and with sponsors. The communal meals and poster sessions offered excellent opportunities for significant interaction amongst conference participants and also for interaction between attendees and the over 20 companies (see below) that were sponsors of the meeting. Dr. Gould highlighted the key role of these sponsors in enabling this very special meeting, and noted that this year featured record sponsorship, with almost triple the number of sponsors relative to the number for last year’s meeting at Asilomar in California. This impressive increase in sponsorship is a reflection of the recent explosion of research and interest in exosomes from many quarters of medicine and science.
Among the themes of this year’s meeting was the growing appreciation for the heterogeneity of exosomes/microvesicles in terms of content, surface markers, size, and function. The similarities between exosomes and viruses were discussed in a number of talks. The brain’s use of exosomes for cell-to-cell communication within the brain, and also to communicate beyond the brain, was highlighted in multiple presentations. One of these suggested the dual promise of extracellular microRNAs in the diagnosis and pathology of Alzheimer’s disease. The role of exosomes in metastasis, carrying information from primary cancer cells to sites of future metastasis, was discussed and presented as further strong support for the century-old “Seed & Soil” hypothesis advanced originally by London surgeon Stephen Paget in 1889 (https://en.wikipedia.org/wiki/Stephen_Paget). Dr. Paget’s original article was titled “Distribution of Secondary Growths in Cancer of the Breast” (Paget, 1889).
One presentation described EVs as epigenetic mediators of systemic communication in murine experimental sepsis and another, by sepsis expert Antonio De Maio, PhD, Professor and Member of the Biomedical Sciences Program at the University of California San Diego, described how phospholipids within EVs may contribute to the activation of target cells. Dr. De Maio, a graduate of the Central University of Venezuela in Caracas, had previously been Associate Professor and Research Director for the Division of Pediatric Surgery at Johns Hopkins, where he had also led the Committee for the Recruitment of Under-Represented Minorities to Graduate Programs. At UCSD, Dr. De Maio is also Director of the Initiative to Maximize Student Diversity Program at the university. At UCSD, Dr. De Maio’s laboratory focuses on the molecular and genetic bases of the response to injury.
Two presentations on tick exosomes and two on bacterial outer membrane vesicles highlighted the broad spectrum of exosome significance throughout the kingdoms of life. An opening night presentation suggested that vesicle-cloaked virus clusters are the optimal units for inter-organismal viral transmission.
The role of exosomes in glioblastoma was the subject of multiple presentations. Janusz Rak, MD, PhD, Senior Scientist in the Child Health and Development Program, and Professor, Department of Pediatrics, McGill University, began the Sunday morning sessions with a talk on the role of EVs in the evolution of glioma-initiating cells. Quantification of cancer EV populations using super-resolution microscopy was another highlight of Sunday morning talks.
ARC is repurposed retrotransposon Gag protein that mediates intercellular RNA transfer in brain
Paul Worley, MD, Professor of Neurology at Johns Hopkins and an expert on the molecular basis of learning and memory, with a focus on cellular mechanisms that support synapse-specific plasticity, opened the Sunday evening session with a highly stimulating discussion of how the neuronal gene ARC encodes a repurposed retrotransposon Gag protein that mediates intercellular RNA transfer. Dr. Worley described evidence suggesting that Gag retroelements have been repurposed during evolution to mediate intercellular communication in the nervous system. Previous work had shown that the neuronal gene ARC is essential for long-lasting information storage in the mammalian brain and mediates various forms of synaptic plasticity. ARC has been implicated in neurodevelopmental disorders. It has been shown that ARC self-assembles into virus-like capsids that encapsulate RNA. Endogenous ARC protein is released from neurons in EVs that mediate the transfer of ARC mRNA into new target cells, where it can undergo activity-dependent translation. Purified ARC capsids are endocytosed and are able to transfer ARC mRNA into the cytoplasm of neurons. ARC exhibits similar molecular properties to retroviral Gag proteins. Evolutionary analysis has indicated that ARC is derived from a vertebrate lineage of Ty3/gypsy retrotransposons, which are also ancestors to retroviruses.
Sensational Tuesday evening
Tuesday evening featured a number of riveting presentations in a sensational session moderated by Xandra Breakefield, PhD, Professor of Neurology, Harvard Medical School, and Geneticist, Massachusetts General Hospital. A presentation on the use of machine learning-assisted histopathology to categorize large oncosomes held the audience spell-bound. Another suggested that EVs serve as delivery vehicles for LINE-1 retrotransposons.
Dr. Tushar Patel, Dean of Research at the Mayo Clinic-Jacksonville and an expert on liver cancer and liver transplants, had opened the session with a discussion of how biological nanoparticles might serve as therapeutic agents.
Other presentations in this session included ones on tools for live monitoring of exosome release from single cells, on the detection of mutant KRAS and TP53 DNA in circulating exosomes from healthy individuals and patients with pancreatic cancer, and on how an infected cell tolerates its viral pathogen using the exosomal pathway.
Beach Boys performance can’t distract ASEMV attendees

Photo: Michael O’Neill
The attendees’ profound interest in exosomes was indicated on the opening evening of the meeting. At the outset, in outlining the logistics of the meeting, ASEMV president Dr. Gould explained that a late-breaking room change for Saturday night’s opening session had been occasioned by concern that the original room might be too noisy due to a performance taking place downstairs by The Beach Boys. The Beach Boys? Many thought that Dr. Gould was joking. But yes, the real Beach Boys were actually playing at a benefit event just downstairs from the ASEMV opening session, and yet, such was the audience’s interest in exosomes that no one moved. This reporter, however, could not resist checking out the iconic band after the opening ASEMV session had ended, and the photo here was taken of The Beach Boys who were indeed playing just downstairs. One of the original band members, Mike Love, was playing keyboard and singing. The Beach Boys performance was the highlight of a gala evening sponsored by Chimes (https://chimes.org/), a Baltimore-based international not-for-profit organization dedicated to assisting people with intellectual and behavioral challenges to achieve their fullest potential. It was an awesome backdrop to what would be an awesome ASEMV meeting.
Nearby International Human Virology meeting features major session on “Exosomes in health & disease”
And one further note is that the Institute for Human Virology (IHV), headed by legendary HIV virologist Dr. Robert Gallo, was holding its 20th International Meeting in the Four Seasons Hotel, right next to the Marriott where the ASEMV meeting was held.
Further indication of the exploding interest in exosomes was that the IHV meeting held a major session on exosomes this year. Titled “Exosomes in Health and Disease,” this session was listed second among nine sessions called out for special attention on the IHV meeting web page (https://www.ihv.org/ihvmeeting/). Areas of emphasis in this session ranged from cytokines in EVs to mechanisms of EVs in viral transmission.
Chairpersons of the IHV exosome session were Robert Gallo, MD, Director, Institute of Human Virology, University of Maryland School of Medicine, US, and Leonid Margolis, PhD, Senior Investigator, National Institute of Child Health and Human Development, US.
Speakers and topics included Xandra Breakefield, PhD, Professor of Neurology, Harvard Medical School / Genetist, Massachusetts General Hospital, “Extracellular Vesicle As Advance Forces in Cancer;” Dr. Margolis, “Not All Soluble Cytokines Are Soluble: Cytokines in Extracellular Vesicles Mediate Cell-Cell Communications;” Fatah Kashanchi, PhD, Former Director of Research, George Mason University, “Presence of HIV-1 RNA in Extracellular Vesicles from HIV-1 cART-Treated Cells;” Ayuko Hoshino, PhD, Instructor of Molecular Biology in Pediatrics, Weill Cornell Medical College, “Exosomal Protein Signatures: Mechanistic Insights and Biomarker Potential;” and Yoel Sadovsky, MD, Executive Director, Magee-Womens Research Institute, University of Pittsburgh, “Placental Exosomes in Maternal-Placental-Fetal Communication and Viral Resistance.”
Sponsors of ASEMV 2018 annual meeting
Sponsors of the ASEMV 2018 annual meeting included Particle Metrix, System Biosciences (SBI), iZON, Caris Life Sciences, nanoView Diagnostics, WAKO, ReNeuron, Norgen Biotek Corporation, Millipore Sigma, Beckman-Coulter, Wyatt Technology, AcouSort, Spectradyne, Fiber Cell Systems, ONI, abcam, Ceres Nano, Nanostics Precision Health, cellex, HansaBioMed Life Sciences, Lonza, and NanoTech.
Reference
Paget S. The distribution of secondary growths in cancer of the breast. The Lancet 133: 571-573. doi: 10.1016/S0140-6736(00)49915-0
Therapeutic exosomes and Huntington’s disease
Extracellular vesicles, specifically exosomes, are currently being explored as therapeutic delivery systems for disease-targeting RNA molecules. In a talk at the ERCC9 conference, Reka A. Haraszti, M.D., a researcher in Dr. Anastasia Khvorova’s group at the University of Massachusetts Medical School, described how exosomes could be used to treat Huntington’s disease, a progressive neurodegenerative disorder. There are currently no effective therapies for this illness, which is caused by a mutation in the Huntingtin gene. Exosomes capable of transporting molecular payloads designed to silence the defective Huntingtin gene represent a potential therapy for this fatal disease.
Comparison of exosome production methods
Technical challenges in the large-scale production of exosomes currently limit their utility for disease treatment. To address this issue, Dr. Haraszti and Dr. Khvorova’s group teamed up with MassBiologics to develop and compare two different exosome production methods for yield and therapeutic efficacy of the exosomes. They utilized Tangential Flow Filtration (TFF) and ultracentrifugation to isolate exosomes from the conditioned media of cultured mesenchymal stem cells.
In TFF, conditioned media is continuously swept along the surface of a filter while a downward pressure is applied to force molecules through the filter. This process is like shaking a sifter to concentrate large particles blocking the holes in the filter, allowing smaller particles to pass through. In contrast, ultracentrifugation works by placing the conditioned media in a column of viscous fluid and spinning rapidly to separate extracellular vesicles in the media by their differing densities.
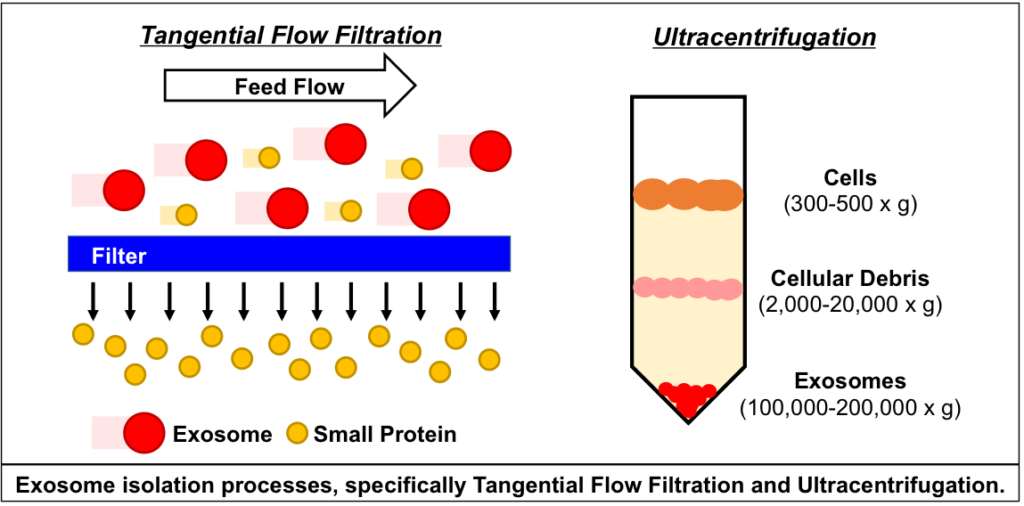
The researchers found that isolation by TFF resulted in 10-100 times more exosomes than ultracentrifugation. TFF-generated exosomes were also more heterogenous and contained 10 times more protein.
Exosomes produced by TFF and ultracentrifugation were also studied for their therapeutic potential in Huntington’s disease. After purification, exosomes were loaded with small interfering RNA (siRNA) molecules that could silence the expression of the mutant Huntingtin gene in target cells that take up the exosomes.
In cell cultures of primary neurons, TFF-generated exosomes showed greater inhibition of Huntingtin expression than those generated by ultracentrifugation. Moreover, TFF-generated exosomes infused into the brains of mice suppressed Huntingtin gene expression in vivo.
Future for therapies using exosomes isolated by Tangential Flow Filtration
The findings of Dr. Haraszti’s group indicate that Tangential Flow Filtration can generate a higher yield of exosomes for clinical use than older methods. Further, TFF-generated exosomes were effective gene therapy agents in experimental models of Huntington’s disease, and hold promise as delivery systems for clinical treatments.
Related Mini-conference
There is an upcoming mini-conference on EV manufacturing and isolation, a topic closely related to the research described here. The in-person conference is in Gainesville, Florida, but a webcast will also be available for those who want to participate remotely.
References
1. Haraszti RA, et al. Loading of extracellular vesicles with chemically stabilized hydrophobic siRNAs for the treatment of disease in the central nervous system. Bio-protocol (2017) 7: e2338. doi: 10.21769/BioProtoc.2338
2. Schwartz L., and Seeley K. Introduction to tangential flow filtration for laboratory and process development applications. Retrieved from https://laboratory.pall.com/content/dam/pall/laboratory/literature-library/non-gated/id-34212.pdf
3. Sunkara V, Woo HK, and Cho YK. Emerging techniques in the isolation and characterization of extracellular vesicles and their roles in cancer diagnostics and prognostics. Analyst (2016) 141: 371-81. doi: 10.1039/c5an01775k
4. Synder Filtration. “Characterization of polymeric, porous membranes: UF/MF & solute rejection measurements.” Retrieved from https://synderfiltration.com/learning-center/articles/membranes/characterization-of-polymeric-porous-membranes/
Extracellular RNA was a hot topic of discussion at Immunology 2016, the annual meeting of the American Association of Immunologists (AAI), held at the Washington State Convention Center in Seattle, Washington May 13-17th, 2016. The National Cancer Institute (NCI) sponsored a symposium on “Extracellular RNA in the Immune System”, co-chaired by Dr. Kevin Howcroft (Division of Cancer Biology, Cancer Immunology, Hematology, and Etiology Branch, NCI) and K. Mark Ansel (University of California San Francisco – your faithful blogger). Four invited speakers presented and participated in lively discussion with an audience of gathered experts and curious newcomers to the field of extracellular RNA.
Dr. Gyongyi Szabo (University of Massachusetts) opened the symposium with a presentation of her laboratory’s work on extracellular vesicles and miRNAs in innate immune cell communication in the liver. Alcohol exposure induces liver inflammation, marked by release of pro-inflammatory cytokines and activation of myeloid cells, including Kupffer cells, the resident macrophages of the liver. In a mouse model, alcohol consumption increased expression of miR-155 in both macrophages and hepatocytes via TLR4 and NFκB-driven transcription. Inhibition or genetic deletion of miR-155 in this model blunted macrophage activation and cytokine production. Exosomes loaded with miR-155 mimetics could be delivered to hepatocytes and other liver cells to correct some of the defects observed in miR-155-deficient animals. Remarkably, endogenous miR-155 and miR-122 were elevated in serum collected after controlled “binge-drinking” in human study subjects, and these exosomes also conveyed information to cultured monocytes, altering their production of TNF and IL-1. Together these data suggest that extracellular communication between hepatocytes and innate immune cells via exosomal miRNAs regulates inflammation in response to alcohol consumption.
The theme of regulation of inflammatory responses by miRNA-containing exosomes was extended by Dr. Ryan O’Connell (University of Utah). His pioneering work on miR-155 and miR-146 demonstrated their opposing roles in inflammatory processes mediated by various cell types in several tissues and disease settings. Recent work in his laboratory showed that both of these miRNAs are released by bone-marrow-derived dendritic cells in a fashion dependent on Rab27 and neutral sphingomyelinase (N-SMase) activity, and that these miRNAs could be exchanged between cells separated by a filter that prevents cell-cell contact. Transferred miR-146a reduced recipient cells’ response to bacterial lipopolysaccharide, a classical innate immune stimulant in vitro and in vivo. In addition, transferred miR-155 was found to directly repress the 3’ UTR of target genes in recipient cells, supporting the possibility that functional miRNA transfer via exosomes could be used as a therapeutic modality for regulating inflammation. Getting these miRNAs to the right cell types in vivo remains an important challenge to bringing this technology to the clinic.
In addition to exosomes, high density lipoprotein (HDL) particles carry miRNAs and other extracellular RNAs in blood. Abnormal pro-inflammatory HDL is associated with systemic lupus erythematosus (SLE). Dani Michell (Vanderbilt University), a postdoctoral fellow in Kasey Vickers’ laboratory, discussed her work, conducted in collaboration with Amy Major’s laboratory, on miRNAs in HDL in SLE. HDL from subjects with SLE contained increased levels of miR-22-3p and miR-192-5p compared with HDL from healthy control subjects. Blocking miR-22 with locked nucleic acid inhibitors in vivo reduced spleen size and interferon production, and affected some clinical features in a mouse model of lupus. Experiments aimed at defining source and recipient cells in this system indicated that monocytes are much better than T lymphocytes at taking up HDL-associated miRNAs. It will be interesting to learn how HDL-associated miRNAs regain gene regulatory function in recipient cells.
The final presentation focused on lymphocytes as source cells for naturally occurring exRNAs in body fluids. Immuno-compromised mice with a mutation that specifically blocks lymphocyte development exhibit altered serum extracellular miRNA profiles. In support of the idea that lymphocytes themselves are an important source of ex-miRNAs, the most reduced exRNA species detected was miR-150, a miRNA highly expressed by lymphocytes. Activated T lymphocytes secrete vesicles that are enriched for tRNA fragments and miRNAs including miR-150. Rigorous purification revealed that these vesicles have characteristics of exosomes, including defined density, size, and protein markers including the tetraspanin CD9. Cellular fractionation also revealed tRNA fragment and miRNA enrichment in membrane fractions containing multivesicular bodies. Whether these extracellular lymphocyte-derived RNAs mediate cell-to-cell communication or not, signal-mediated reduction of cellular miRNAs certainly alters gene regulation in activated T lymphocytes. Thus, exRNA secretion may have important roles in regulating inflammatory processes in both source and recipient cells.
These topics will certainly remain on the mind of immunologists that attended the exRNA symposium — at least until Immunology 2017, to be held in Washington DC next May.
Mark your calendars for two upcoming conferences on extracellular vesicles. In early May, the International Society for Extracellular Vesicles (ISEV) will hold its fourth annual meeting in Rotterdam, the Netherlands. If you can’t make it to Europe, the American Society for Exosomes and Microvesicles is holding its annual meeting in northern California in October. Hope to see you all there!