Alzheimer’s disease (AD) is the most common form of dementia, a general term for loss of memory and other intellectual abilities serious enough to interfere with daily life. AD accounts for 60 – 80% of dementia cases, and is the sixth leading cause of death in the United States. The greatest known risk factor for AD is increasing age; most people with AD are 65 and older. As AD is a progressive disease, and dementia symptoms gradually worsen over several years, costs increase in parallel with dementia and behavioral disturbances, which require increased caregiving time and intensity. The cost of AD in the United States today is estimated to be $100 billion each year; the greatest costs are for long-term care by health care professionals and institutionalization, with significant family costs for direct caregiving and lost earnings. Current AD treatments do not stop disease progression, but they can slow the progression of dementia symptoms and improve quality of life for those with AD and their caregivers, and thus provide tremendous economic and social benefits. However, there is no clinical diagnostic test for AD, nor any that distinguishes early AD from age-related dementia. Such a tool would be invaluable in guiding clinicians towards early interventional efforts.
The existence of extracellular RNAs (exRNAs) in biofluids represents a fertile molecular landscape from which diagnostic and prognostic biomarkers may be accessed, characterized, and exploited. The mission of the Extracellular RNA Communication Program (ERCP) is to further basic and translational studies on exRNAs in a wide range of biofluids and human diseases. Our studies are focused on the identification of exRNAs in cerebrospinal fluid (CSF) that can be used as biomarkers for AD. CSF is produced in the brain and circulates through the ventricles and spinal cord, providing a buffer against chemical imbalances and mechanical injury, and a means to clear metabolic waste from the brain. The brain produces ~500 mL of CSF per day, with ~150 mL present at any time. Biological components in CSF provide a sensitive indicator of changes in the brain. For example, prominent protein biomarkers for AD include beta-amyloid and tau. Combined measures of beta-amyloid fragment and phosphorylated tau show high sensitivity for AD (90%), but low specificity (64%), which limits their clinical utility. To date there are no clinical biomarkers that can predict the onset of AD, distinguish AD from mild cognitive impairment, or distinguish the individual stages of AD (latent, mild, moderate, severe). Further, there are no studies to date to examine the utility of exRNAs in CSF from living human donors as AD biomarkers.
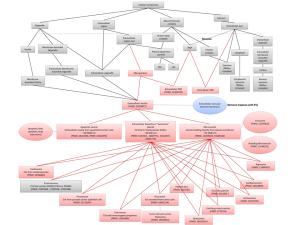
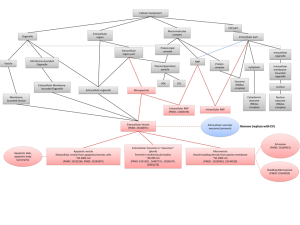
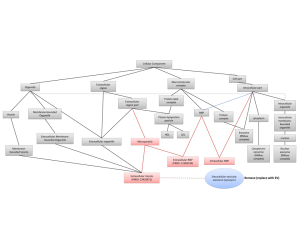
MicroRNAs (miRNAs) are one example of exRNAs that are increasingly found in circulating biofluids such as CSF, plasma, serum, and placental tissue, where their expression is correlated with several diseases including brain injury (traumatic brain injury, ischemia), degenerative disease (multiple sclerosis and AD), and mental health disorders (bipolar disorder). MiRNAs are members of the non-protein-coding family of RNAs that serve as regulators of post-transcriptional gene expression. Importantly, miRNAs are stable in circulating fluids, presumably because they are contained within vesicles or ribonucleoprotein complexes, which likely affords them protection against ribonuclease digestion. MiRNAs bind to specific proteins to form RNA-induced silencing complexes (RISCs), and together they can bind messenger RNA (mRNA) to either repress mRNA translation, or degrade mRNA transcripts, to ultimately regulate the expression of target proteins in the cell. Given that AD is generally characterized by an over-accumulation of proteins, we proposed that a loss of miRNAs might lead to overexpression and accumulation of proteins in AD brain.
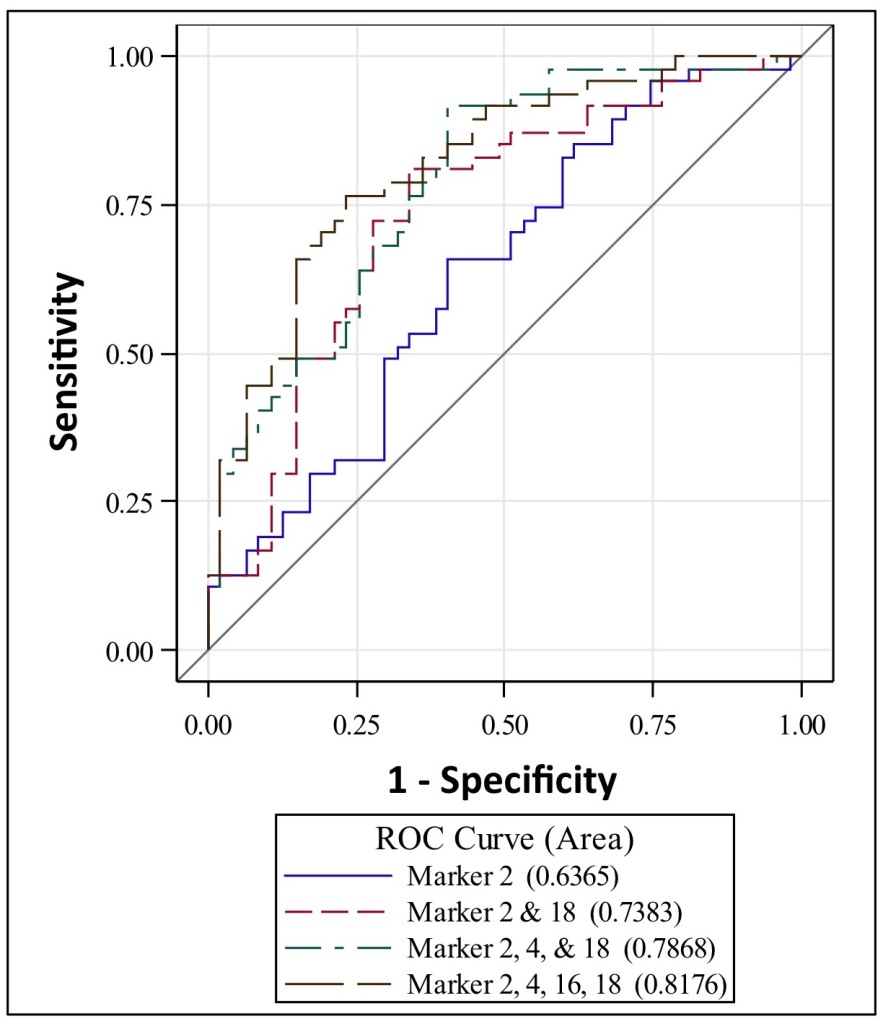
To examine this hypothesis, we obtained miRNAs in CSF from 47 AD and 47 control living human donors from the Oregon Alzheimer’s Disease Center (OADC). The OADC uses standardized methods for CSF collection that are consistent with other dementia research centers. Total RNA was isolated from CSF, and miRNA detection was performed using PCR based methods that are in widespread clinical use. Each miRNA cycle threshold (Ct) value was normalized to U6 snRNA internal controls (ΔCt). Of 332 CSF miRNAs, we found that 19 had significant differential expression between AD and control, and these 19 miRNAs were detected less often in AD versus control CSF. Logistic regression models and receiver operating characteristic (ROC) curves were then used to evaluate combinations of the 19 top-performing miRNA to accurately classify subjects into AD and control. Figure 1 shows that area under the ROC curves demonstrate 63% classification accuracy for 1 miRNA, 73% for two miRNAs, and 81% for 4 miRNAs. Thus, combinations of miRNAs show better discrimination between AD and control CSF. Together, our studies support that miRNAs in human CSF show potential utility as biomarkers for AD, and suggest that CSF exRNAs may be used as biomarkers for other dementia and neurodegenerative diseases.